Abstract
Background: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive hematologic malignancy with unique clinical presentation often involving skin, bone marrow, and lymph nodes, as well as central nervous system (CNS) involvement in 20-30% of patients with BPDCN (Pemmaraju et al Blood June 2021). In recent years, the development of novel agents targeted against CD123 has changed the treatment landscape of BPDCN, yet relapses (both systemic and CNS) still frequently occur in the setting of CD123-directed monotherapy (Pemmaraju et al NEJM April 2019). Targeted therapy with BCL-2 inhibitor venetoclax has also demonstrated activity against BPDCN alone or in combination with chemotherapy (Montero et al Cancer Discovery 2017; DiNardo et al Am J Hematol 2018; Pemmaraju et al NEJM Feb 2019). However, given the lack of blood-brain-barrier penetration of most available targeted agents, multi-agent regimens with prophylactic CNS therapy administered with serial lumbar punctures with intrathecal (IT) chemotherapy, as used in treatment for ALL, have also been utilized for BPDCN. We sought to analyze outcomes of our most commonly administered cytotoxic chemotherapy backbone regimen for patients with BPDCN, Hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone alternating with methotrexate/ARA-C (HCVAD) (Kantarjian etal JCO 2000).
Methods/Patients: We conducted a single-institution, retrospective analysis of patients with BPDCN (n=100), evaluating complete remission (CR) and overall survival (OS) outcomes of those who received frontline HCVAD-based vs non-HCVAD based regimens. HCVAD-based regimens were administered in n=35 and included: HCVAD n=23; HCVAD+venetoclax n=4; mini-HCVAD+venetoclax+/-enasidenib n=2; HCVAD+bortezomib n=2; modified HCVAD n=1, and HCVAD+ SL-401 (tagraxofusp-erzs) +/-venetoclax n=3.
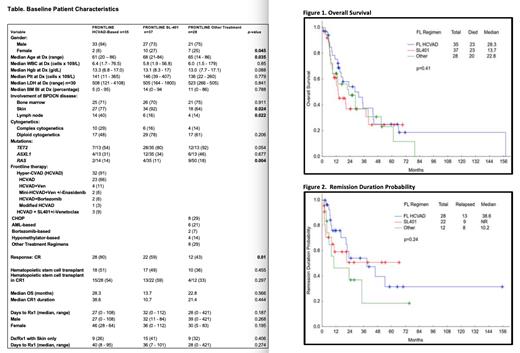
Results: Baseline characteristics for patients are divided into 3 separate groups for comparison: Group 1: patients who received frontline HCVAD-based regimens (n=35) vs Group 2: frontline SL-401 (n=37) vs Group 3: other treatment regimens (n=28) as listed in detail in Table. Frontline HCVAD-based patients had a higher prevalence of male sex (94% vs 73% vs 75%, p=0.045), younger median age (61 vs 68 vs 65 years, p=0.035), and higher degree of lymph node involvement (40% vs 16% vs 14%, p=0.022); SL-401 patients had higher degree of skin involvement (92% vs 77% vs 64%, p=0.024); Other treatment regimens patients had a higher percentage of RAS mutations (18% vs 14% vs 11%, p=0.004). Importantly, HCVAD-based regimens yielded higher CR responses (80% vs 59% vs 43%, p=0.01). There was no statistically significant difference in OS among therapy cohorts (median OS, 28.3 vs 13.7 vs 22.8 months) (Figure 1). There was no statistically significant difference in remission duration probability among treatment groups (38.6 vs NR vs 10.2 months; p=0.24) (Figure 2). Hematopoietic stem cell transplant (HSCT) was performed in 51% of those receiving frontline HCVAD-based vs 49% of frontline SL-401 vs 38% other treatment regimen patients (p=0.455).
Conclusions: Despite significant progress in CD123- and BCL-2-targeted therapy approaches in BPDCN, most patients are not cured outside of HSCT, and CNS relapses are now emerging commonly in the modern targeted therapy era of BPDCN. Combination approaches with both targeted and cytotoxic chemotherapy incorporating prophylactic CNS-directed therapy are urgently needed in this disease. These results establish high rates of CR for patients treated with frontline HCVAD, and establish a baseline role still, even in the modern targeted therapy era, for cytotoxic chemotherapy regimens in the treatment of BPDCN; in particular, confirming the role for HCVAD-based chemotherapy for BPDCN. Further studies are ongoing to establish the clinical feasibility, safety, and activity of doublet and triplet combinations of targeted therapies with cytotoxic agents with the goal of durable long-term remissions. Our group is actively investigating triplet combination with HCVAD as a comprehensive combination therapy protocol for BPDCN that includes three most active regimens: CD123, BCL-2 and ALL-based cytotoxic approach with SL-401/venetoclax/HCVAD in addition to IT chemotherapy for CNS prophylaxis (NCT04216524).
Pemmaraju: Cellectis S.A. ADR: Other, Research Funding; Samus: Other, Research Funding; LFB Biotechnologies: Consultancy; Plexxicon: Other, Research Funding; Aptitude Health: Consultancy; Roche Diagnostics: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; CareDx, Inc.: Consultancy; Springer Science + Business Media: Other; ImmunoGen, Inc: Consultancy; Bristol-Myers Squibb Co.: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; DAVA Oncology: Consultancy; MustangBio: Consultancy, Other; Daiichi Sankyo, Inc.: Other, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Sager Strong Foundation: Other; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Affymetrix: Consultancy, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Pacylex Pharmaceuticals: Consultancy. Sasaki: Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Khoury: Kiromic: Research Funding; Angle: Research Funding; Stemline Therapeutics: Research Funding. Jain: Pharmacyclics: Research Funding; AbbVie: Honoraria, Research Funding; Servier: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Research Funding; TG Therapeutics: Honoraria; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Janssen: Honoraria; Beigene: Honoraria; Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding. Borthakur: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Protagonist: Consultancy; University of Texas MD Anderson Cancer Center: Current Employment; Ryvu: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding. Ravandi: Xencor: Honoraria, Research Funding; AstraZeneca: Honoraria; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Prelude: Research Funding; Amgen: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding. Daver: Pfizer: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Hanmi: Research Funding; Trovagene: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; Trillium: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Kadia: Sanofi-Aventis: Consultancy; BMS: Other: Grant/research support; Jazz: Consultancy; Novartis: Consultancy; Liberum: Consultancy; Pulmotech: Other; Aglos: Consultancy; AstraZeneca: Other; Cure: Speakers Bureau; Pfizer: Consultancy, Other; Cellonkos: Other; Dalichi Sankyo: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Amgen: Other: Grant/research support; Genentech: Consultancy, Other: Grant/research support; Ascentage: Other; Genfleet: Other; Astellas: Other. DiNardo: Agios/Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Novartis: Honoraria; Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Qazilbash: Bristol-Myers Squibb: Other: Advisory Board; Oncopeptides: Other: Advisory Board; Biolline: Research Funding; NexImmune: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; Janssen: Research Funding. Konopleva: Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Sanofi: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Cellectis: Other: grant support; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Forty Seven: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Stemline Therapeutics: Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; KisoJi: Research Funding. Kantarjian: Immunogen: Research Funding; Ascentage: Research Funding; Astra Zeneca: Honoraria; BMS: Research Funding; Amgen: Honoraria, Research Funding; NOVA Research: Honoraria; Daiichi-Sankyo: Research Funding; KAHR Medical Ltd: Honoraria; AbbVie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Ipsen Pharmaceuticals: Honoraria; Astellas Health: Honoraria; Aptitude Health: Honoraria; Novartis: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal